Definition of Radioactivity and Radiation: Unstable atomic nuclei will spontaneously decompose to form nuclei with a higher stability. The decomposition process is called radioactivity. The energy and particles which are released during the decomposition process are called radiation. When unstable nuclei decompose in nature, the process is referred to as natural radioactivity. When the unstable nuclei are prepared in the laboratory, the decomposition is called induced radioactivity.
Answer: There are three major types of natural radioactivity: 
- Alpha Radiation
Alpha radiation consists of a stream of positively charged particles, called alpha particles, which have an atomic mass of 4 and a charge of +2 (a helium nucleus). When an alpha particle is ejected from a nucleus, the mass number of the nucleus decreases by four units and the atomic number decreases by two units. For example:
23892U -> 42He + 23490Th
The helium nucleus is the alpha particle.
- Beta Radiation
Beta radiation is a stream of electrons, called beta particles. When a beta particle is ejected, a neutron in the nucleus is converted to a proton, so the mass number of the nucleus is unchanged, but the atomic number increases by one unit. For example:
23490 -> 0-1e + 23491Pa
The electron is the beta particle.
- Gamma Radiation
Gamma rays are high-energy photons with a very short wavelength (0.0005 to 0.1 nm). The emission of gamma radiation results from an energy change within the atomic nucleus. Gamma emission changes neither the atomic number nor the atomic mass. Alpha and beta emission are often accompanied by gamma emission, as an excited nucleus drops to a lower and more stable energy state.
Alpha, beta, and gamma radiation also accompany induced radioactivity. Radioactive isotopes are prepared in the lab using bombardment reactions to convert a stable nucleus into one which is radioactive. Positron (particle with the same mass as an electron, but a charge of +1 instead of -1) emission isn't observed in natural radioactivity, but it is a common mode of decay in induced radioactivity. Bombardment reactions can be used to produce very heavy elements, including many which don't occur in nature.


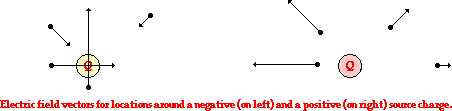
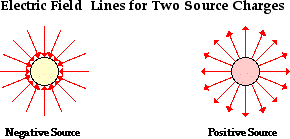
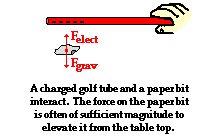 push or pull exerted upon an object as a result of an interaction with another object. The interaction is the result of electrical charges and thus it is called an electrical force.
push or pull exerted upon an object as a result of an interaction with another object. The interaction is the result of electrical charges and thus it is called an electrical force.

 shared by the atoms. The clouds become distorted, with the electrons having the greatest probability of being found closest to the more electron-greedy atom. The bond is said to be a
shared by the atoms. The clouds become distorted, with the electrons having the greatest probability of being found closest to the more electron-greedy atom. The bond is said to be a